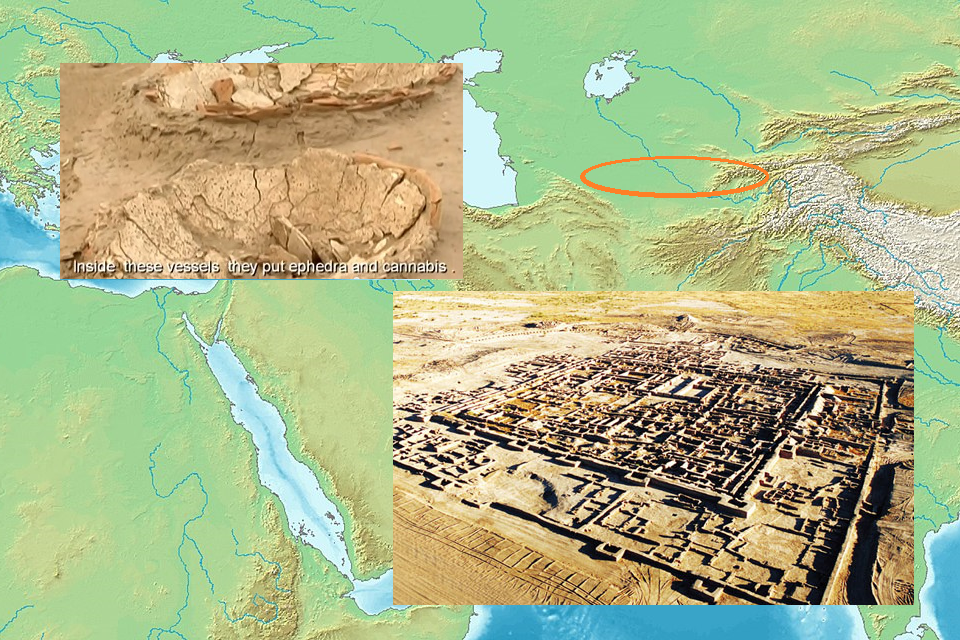
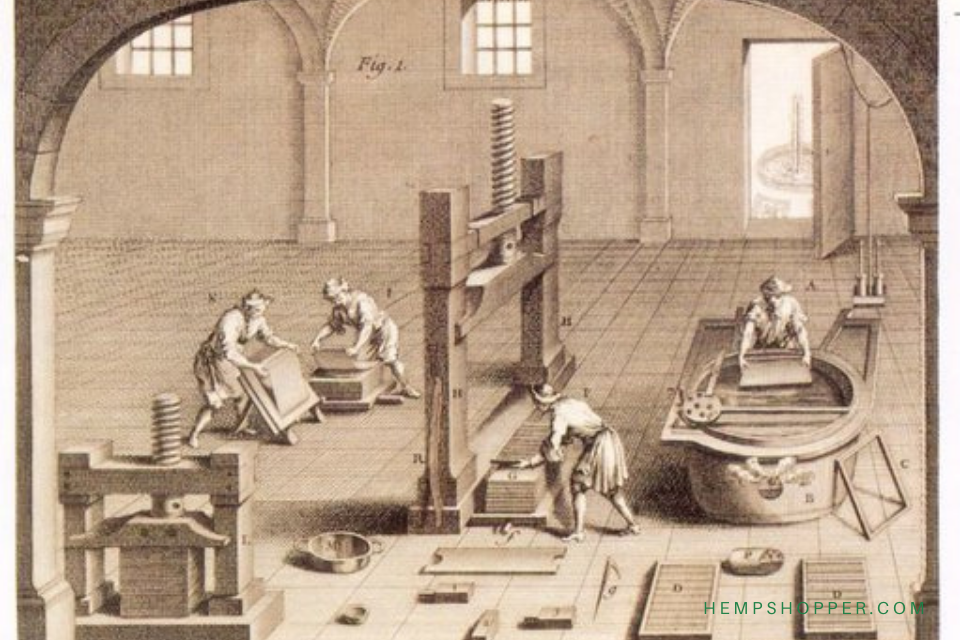
1906: The Pure Food and Drug Act makes cannabis legally available

1906: The Pure Food and Drug Act makes cannabis legally available.
Federal regulation of drugs began with the Pure Food and Drug Act of 1906. The FDA could sample imported foods for adulteration, misbranding, or health hazards. The Act transformed the Division of Chemistry from a scientific bureau to a regulatory agency.
The Pure Food and Drug Act was initially concerned with ensuring products were labelled correctly. Later efforts were made to outlaw certain products that were not safe, followed by efforts to outlaw products which were safe but not effective. For example, there was an attempt to outlaw Coca-Cola in 1909 because of its excessive caffeine content; caffeine had replaced cocaine as the active ingredient in Coca-Cola in 1903.
In addition to caffeine, the Pure Food and Drug Act required that drugs such as alcohol, cocaine, heroin, morphine, and cannabis, be accurately labelled with contents and dosage. Previously many drugs had been sold as patent medicines with secret ingredients or misleading labels.
Cocaine, heroin, cannabis, and other such drugs continued to be legally available without prescription as long as they were labelled. The states decided over public health laws and cannabis was considered medicine and it was sold in pharmacies. There was nothing called illegal drug at that time, on a national level.
That law made the manufacture of an adulterated or misbranded drug a misdemeanour, carrying a punishment not to exceed a $200 fine and/or one year in prison for the first conviction.
The main objective of the law was ensure correct labeling, reducing adulterated food and drugs and informing consumers to increase safety for products involved in international or interstate commerce, but, eventually, this can be consider the first foundation for cannabis prohibition, which president Theodore Roosevelt signed into law on June 6 of that year.
However, the Act did not require government clearance yet for new drugs and companies were still permitted to market new drugs without first proving their safety or effectiveness.
The new law did not become effective until January 1, 1907, when Dr.Wiley, known as the “Father of the Pure Food and Drugs Act of 1906″, had been conducting enough laboratory studies on food adulteration, as part of his job with the Department of Agriculture since 1883. Dr Wiley created the “Poison Squad” in 1902, which was a group of 20 healthy men who were chosen to eat foods containing suspected poisons that were dyes or preservatives.
-SMITH DE WAAL, C., ROBERTS, C., OLUNKETT, D. (2013): The Legal Basis for Food Safety Regulation in the USA and EU in Food borne Infections and Intoxications. Chapter 36. Academic Press. -BRODY, T: Origins of the Federal Food, Drug and Cosmetic Act and Its Amendments. Chapter 33 in Clinical Trials, Second edition: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines. Elsevier, 2016. -History, Art and Archives. United States House of Representatives, Historical Highlights -US Food and Drug Administration. fda.gov Research and text © Hempshopper Amsterdam.


 Hempshopper Amsterdam
Hempshopper Amsterdam